http://www.microrao.com/micronotes/anatomy.pdf
Our silver ions will have to pierce the cell wall of the bacterium. The most important component of most bacteria cell walls is an endotoxin called lipopolysaccharide. These endotoxin molecules carry a negative charge.
The positively charged single atoms of silver will eagerly bind with them, turning off their function. The destruction of these bacteria explains the Herxheimer effect experienced by people using colloidal (ionic) silver for the first time infected by one of these organisms.
There needs to be more clarity about ionic versus particulate colloidal silver. We measure the concentration or strength of colloidal silver by its conductivity. It is measured in Microsiemens. One Microsiemen of conductivity equals one ppm of ionic colloidal silver. The presence of ions causes conductivity in a liquid. Without ions, there is no conductivity. It is an insulator. No current will flow. So, the same measure that we use to denote the strength of a colloidal silver solution is, by definition, the number of ions present. An ion is a single atom of an element, in this case, silver, with a single electron, either missing or added. In the case of silver, the outer valence bond band contains only one electron. It is relatively easy for silver to become an ion. Its outer electron is only loosely held. It becomes a positive ion looking for another element with which it can share an electron. Your body is one huge electrical factory with all sorts of elements very busily combining and disassociating. All of this happens cut with ions. If you go into a hospital where they have to find out what’s wrong with you, one of the first things they will do is check your electrolytes. An electrolyte is a substance that dissociates into ions in solution and acquires the capacity to conduct electricity. Sodium, potassium, chloride, calcium, and phosphate are examples of electrolytes, informally known as lytes.
When we make colloidal silver, it is the process called electrolysis. Initially, everything that leaves the anode is an ion. If we try to make too many leave the anode simultaneously, they will start combining and discharge, forming particles of silver with 2, 3, or a thousand or more atoms and lose their charge. If they become large enough, they will fall out of suspension and form little black dots at the bottom of the glass. There is some evidence that it’s a good idea for intestinal problems to have some particles make it down to your intestine. As long as they are small, they will pass through, and the ones that do make it into your bloodstream will be filtered out by your kidneys and pass in your urine within 48 hours. So, we need some way to judge when we have made the particles to an acceptably small size to stop the process at that point. That coincides with the point at which we will have reached the maximum sustainable ionic strength in ppm. Before the advent of lasers, colloidal silver makers used a flashlight beam. Shining the light down through the solution formed a cone-shaped rainbow effect caused by the particles’ diffraction. That is called the Tyndall effect. Lacking this, most people would continue the process until the entire solution had a yellow tinge.
Unfortunately, by the time the whole solution becomes yellow, the particles are more than 50 nm. Continued daily use, which is the case with many people, of this type of colloidal silver could lead to argyria. It is mainly a cause of concern for very light-skinned people. A laser enables us to start seeing particles of a size less than 50 nm. Unfortunately, virtually all the cheap laser pointers from China have no regulation. The power of the laser in these devices will vary directly with the condition of the battery. That is not acceptable in a piece of test equipment. Atlasnova went to Taiwan and secured a line of laser pointers that are tightly regulated. The least expensive of these is the one supplied with our various colloidal silver generator kits. It maintains an exact power until the battery is completely dead. The scattering effect causes the phenomenon which produces the red line in the solution with the laser. Many suppliers still referred to this as a Tyndall effect.
Harvard has just released a study on developing an “AAD (active adhesive dressing). They have patented it, and I’m sure it will be a real moneymaker for them.
https://advances.sciencemag.org/content/advances/5/7/eaaw3963.full.pdf
particles or ions, which is best?
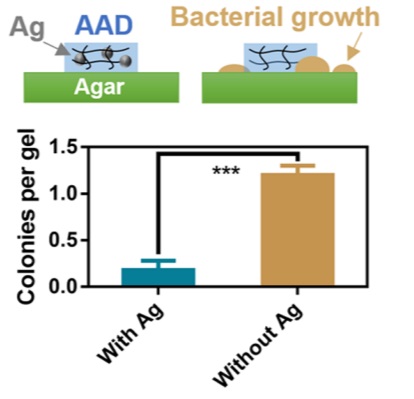
This study is very well conducted, as you would expect from Harvard. The addition of silver nanoparticles is proven to have increased the antibiotic effect of the hydrogel. I refer you to the following quote from the article discussing the nanoparticles in the hydrogel:
“To test the antimicrobial function of HN, we analyzed bacterial growth on agar plates in the presence of the adhesive hydrogels with and without AgNPs (fig. S1). AgNP-containing HN-Ag effectively inhibited bacterial growth (Fig. 2E). To examine whether the AgNPs were lost from HN-Ag over time, we performed A released study. We found no AgNPs that leaked from the hydrogels, Indicating That the Antimicrobial Function of HN Ag Relied on the Release of Silver Ions, Not of Nanoparticles, from the Gels.”
Here’s a picture of the process.
How about making a bandage emulating a blister, covering the whole wound, filling it with 50 PPM EIS?
HOUSTON – (July 11, 2012) – Rice University researchers have settled a long-standing controversy over the mechanism by which silver nanoparticles, the most widely used nanomaterial in the world, kill bacteria.
Their work comes with a warning: Use enough. If you don’t kill them, you make them stronger.
Scientists have long known that silver ions, which flow from nanoparticles when oxidized, are deadly to bacteria. Silver nanoparticles are used just about everywhere, including in cosmetics, socks, food containers, detergents, sprays and a wide range of other products to stop the spread of germs.
But scientists have also suspected silver nanoparticles themselves may be toxic to bacteria, particularly the smallest of them at about 3 nanometers. Not so, according to the Rice team that reported its results this month in the American Chemical Society journal Nano Letters.
In fact, when the possibility of ionization is taken away from silver, the nanoparticles are practically benign in the presence of microbes, said Pedro Alvarez, George R. Brown Professor and chair of Rice’s Civil and Environmental Engineering Department.

“You would be surprised how often people market things without a full mechanistic understanding of their function,” said Alvarez, who studies the fate of nanoparticles in the environment and their potential toxicity, particularly to humans. “The prefix ‘nano’ can be a double-edged sword. It can help you sell a product, and in other cases it might elicit concerns about potential unintended consequences.”
He said the straightforward answer to the decade-old question is that the insoluble silver nanoparticles do not kill cells by direct contact. But soluble ions, when activated via oxidation in the vicinity of bacteria, do the job nicely.
To figure that out, the researchers had to strip the particles of their powers. “Our original expectation was that the smaller a particle is, the greater the toxicity,” said Zongming Xiu, a Rice postdoctoral researcher and lead author of the paper. Xiu set out to test nanoparticles, both commercially available and custom-synthesized from 3 to 11 nanometers, to see whether there was a correlation between size and toxicity.
“We could not get consistent results,” he said. “It was very frustrating and really weird.”
Xiu decided to test nanoparticle toxicity in an anaerobic environment – that is, sealed inside a chamber with no exposure to oxygen — to control the silver ions’ release. He found that the filtered particles were a lot less toxic to microbes than silver ions.
Working with the lab of Rice chemist Vicki Colvin, the team then synthesized silver nanoparticles inside the anaerobic chamber to eliminate any chance of oxidation. “We found the particles, even up to a concentration of 195 parts per million, were still not toxic to bacteria,” Xiu said. “But for the ionic silver, a concentration of about 15 parts per billion would kill all the bacteria present. That told us the particle is 7,665 times less toxic than the silver ions, indicating a negligible toxicity.”
“The point of that experiment,” Alvarez said, “was to show that a lot of people were obtaining data that was confounded by a release of ions, which was occurring during exposure they perhaps weren’t aware of.”
Ions, not particles, make silver toxic to bacteria
Journal of Applied MicrobiologyVolume 123, Issue 5
Here is an authoritative review documenting the effectiveness of silver. There is all the evidence that is needed here and none of it can be characterized as “anecdotal.”:
https://pubmed.ncbi.nlm.nih.gov/22784602/
Of particular interest at this time would be the information of action against viruses:
“Antiviral activity of natural mineral silver in a variety of forms including colloidal silver has been demonstrated through nearly three decades of medical research (Fig. 2). It has been reported that silver can stop different types of viruses from replicating by merely binding to them. Recent research demonstrates that silver is so powerfully effective against viruses that it even stops the deadly HIV virus from infecting human cells. The vital requirement in order to exhibit such powerful antiviral activity is the size of the silver particles. Nanoparticles of size ranging from 1 to 100 nm are efficient as smaller size leads to more interaction and inhibition of viruses (Galdiero et al. 2011; Khandelwal et al. 2014). Silver nanoparticles undergo a size‐dependent interaction with HIV‐1 virus and nanoparticles in the size range of 1–10 nm were able to attach to the virus. The interaction is via preferential binding of AgNPs to the gp120 glycoprotein knobs which bear the exposed sulfur residues and inhibit the virus from binding to host cells in vitro (Elechiguerra et al. 2005).”
Careful reading will reveal that our quest to maximize the ionic portion of what we make has been the correct approach.
Since I started this thread in 2014, we have seen one study after another for this or that latest silver nanoparticles. All produced by some unique process that is patented. All of these studies show the wonderful effects of their particular substance against infectious agents. I have believed that the effectiveness of these various wonderful new nanosilver concoctions was that once they enter our body, they eventually come into contact with oxygen, resulting in the release of silver ions. Single silver’ ions with a negative charge. Here is a report that exposes the entire scam:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5855666/
I quote from the “Summary and Conclusions.”
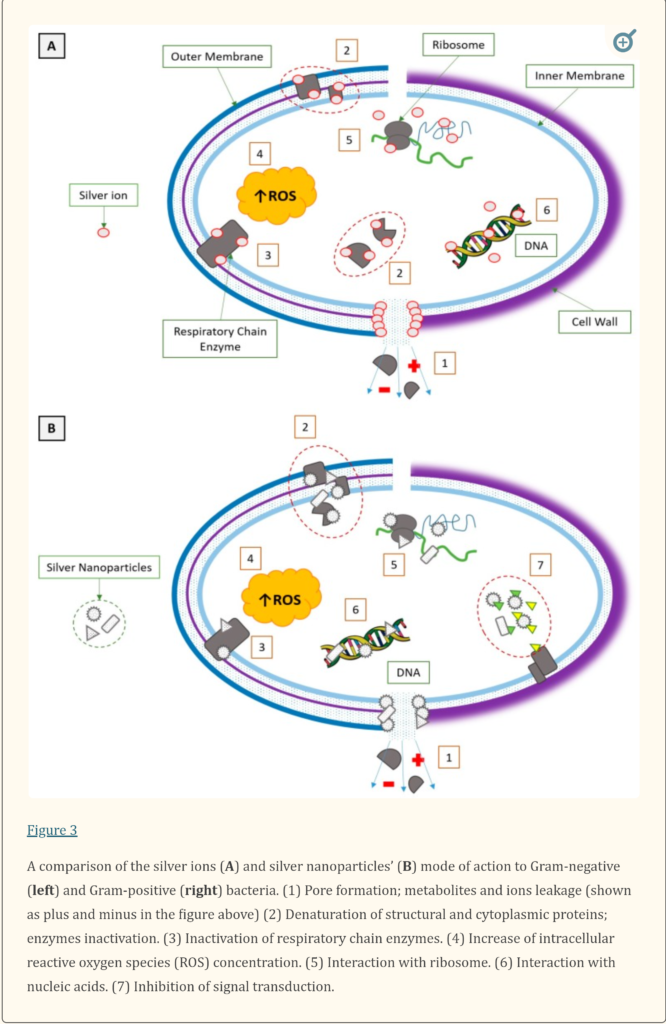
“We presented the similarities and differences in the mode of silver action on Gram-positive and Gram-negative bacteria. We noticed a gap in knowledge about the molecular mechanism of bacteria, both Gram-positive and Gram-negative, to silver nanoparticles. If we assume that silver nanoparticles are silver ions source, it is possible that the molecular mechanism of bacterial resistance is analogue to mechanism described for Ag+. If there is another way of antibacterial toxicity of silver nanoparticles, it is likely that different mechanisms of resistance to silver nanoparticle exist, for both Gram-negative and Gram-positive bacteria. The variety of silver nanoforms causes that every product with silver nanoparticles should be considered separately as a compound with different physico-chemical properties, different mode of action and different mechanisms of resistance.”
#398
So the question I ask is if silver ions are more effective at eliminating bacteria than silver nanoparticles, why is all this time and money being spent on developing so many different nanoparticles?
You can’t patent silver ions. Moreover, everybody can make their own.
Money money money.
Silver nanoparticles versus silver ions?
Li W.-R., Sun T.-L., Zhou S.-L., Ma Y.-K., Shi Q.-S., Xie X.-B., Huang X.-M. A comparative analysis of antibacterial activity, dynamics and effects of silver ions and silver nanoparticles against four bacterial strains. Int. Biodeterior. Biodegrad. 2017;123:304–310. doi: 10.1016/j.ibiod.2017.07.015. [CrossRef] [Google Scholar]
https://www.sciencedirect.com/science/article/pii/S0964830517306406
Although both silver ions and silver nanoparticles (AgNPs) have perfect antibacterial activity, but it was assumed that AgNPs have stronger activity than that of silver ions. In this study, we make a comparative analysis of activity, dynamics, and effects of silver ions and two types of AgNPs against four bacterial strains. The minimum inhibitory concentrations (MICs) of silver ions, AgNPs (I) and AgNPs (II) were 0.5, 1 and 2 μg/mL against E. coli, 1, 2 and 8 μg/mL against P. aeruginosa, 1, 2 and 4 μg/mL against S. aureus, and 1, 2 and 2 μg/mL against S. epidermidis respectively. This experimental results showed that Ag+ have stronger antibacterial activity than that of AgNPs (I) and AgNPs(II). Antibacterial dynamic curves revealed all the silver ions, AgNPs (I), and AgNPs (II) prolonged the growth lag phase of all four bacteria in a concentration-dependent manner. Furthermore, transmission electronic microscopy (TEM) observation showed that a major part of bacterial cells treated with 2 μg/mL of silver ion and AgNPs were destroyed within 5 h. The transmission electron microscopy (TEM) observation indicated that all the silver ions, AgNPs (I), and AgNPs (II) can induce severe damage in bacterial cells. The flagella of bacteria were damaged or even eliminated, which would cause movement disorders. Many holes or gaps were observed on cell surfaces, which would cause the leakage of cytoplasm and macromolecules, and leading to cell death at last. Our results suggested that silver ions have similar action mode and slightly better antibacterial activity than that of AgNPs against bacterial cells.
Here is the most up to date and authoritative information available as to the use of silver as medicine. Just click on the link to view the pdf of the entire article.
https://doi.org/10.1111/jam.13525
We have seen one study after another for this or those latest silver nanoparticles. All are produced by some unique process that is patented. These studies show their particular substance’s excellent effects against infectious agents. The effectiveness of these beautiful new nanosilver concoctions was that once they enter our body, they eventually come into contact with oxygen, releasing silver ions. Single silver ions with a negative charge. Here is a report that exposes the entire scam:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5855666/
I quote from the “Summary and Conclusions.”
“We presented the similarities and differences in the mode of silver action on Gram-positive and Gram-negative bacteria. We noticed a gap in knowledge about the molecular mechanism of bacteria, both Gram-positive and Gram-negative, to silver nanoparticles. If we assume that silver nanoparticles are silver ions source, it is possible that the molecular mechanism of bacterial resistance is analogue to mechanism described for Ag+. If there is another way of antibacterial toxicity of silver nanoparticles, it is likely that different mechanisms of resistance to silver nanoparticle exist, for both Gram-negative and Gram-positive bacteria. The variety of silver nanoforms causes that every product with silver nanoparticles should be considered separately as a compound with different physico-chemical properties, different mode of action and different mechanisms of resistance.”